
Every day, pharmacists hand out millions of generic drugs. They’re cheaper, widely available, and approved by the FDA. But here’s the problem: when something goes wrong with a generic medication, pharmacists are often the first-and sometimes the only-person who notices. Yet, most never report it.
Why Pharmacists Are Key to Generic Drug Safety
Pharmacists don’t just fill prescriptions. They see what happens after the pill is taken. A patient comes back saying their blood pressure meds aren’t working like they used to. Another complains of dizziness after switching from one generic brand to another. These aren’t random quirks. They’re red flags.
The FDA defines therapeutic inequivalence as when a generic drug doesn’t perform the same way in real life as the brand-name version-even if it passed lab tests. This isn’t theoretical. In 2022, the FDA received over 28,000 reports involving generic drugs, up from just over 12,000 in 2015. That’s a 131% jump. And a surprising number of those signals came from pharmacists who noticed patterns across multiple patients.
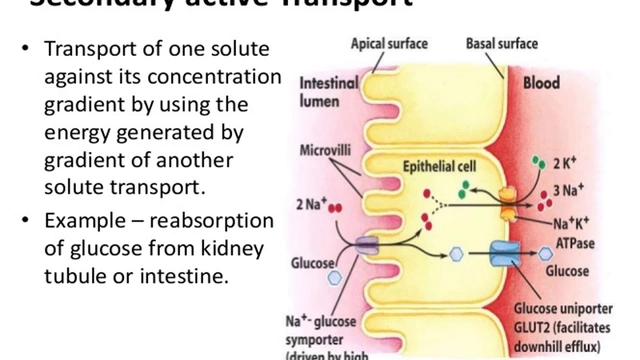
Unlike doctors who write prescriptions, pharmacists are the last checkpoint before the patient swallows the pill. They see the label, the manufacturer, the lot number. They know if the patient switched from one generic to another last week. They’re the ones who hear, “This one doesn’t feel right.” That’s real-world evidence. And it’s exactly what the FDA needs to catch problems that clinical trials miss.
What Exactly Should You Report?
You don’t need to be certain. You just need to suspect.
The FDA asks for reports when there’s a reasonable chance the drug caused the issue. That includes:
- Life-threatening reactions
- Hospitalizations
- Permanent disability
- Birth defects
- Unexpected side effects
- Therapeutic failure-like a seizure returning after switching to a new generic epilepsy drug
Therapeutic inequivalence is the big one. It’s when a patient does fine on Brand X, then switches to Generic A, and suddenly their condition worsens. No new illness. No change in dose. Just a different manufacturer. That’s not a coincidence. It’s a signal.
The FDA’s MedWatch Form 3500, updated in January 2023, now has a specific checkbox for “generic drug concern.” You can pick whether it’s about therapeutic inequivalence, manufacturing quality, or labeling issues. That’s new. And it matters.
Why Don’t More Pharmacists Report?
If pharmacists are so critical to spotting these issues, why are they responsible for less than 3% of all reports?
Time is the biggest blocker. In a busy community pharmacy, you’re juggling refill requests, insurance calls, counseling patients, and managing inventory. Filling out a detailed report takes 15-20 minutes. Most don’t have it.
Then there’s confusion. Many pharmacists aren’t sure what counts as reportable. Is it just severe reactions? What if the patient just feels “off”? What if you don’t know which generic brand they took? The FDA says: report anyway. You don’t need to be 100% sure. You just need to report the pattern.
And here’s the tricky part: attribution. Generic manufacturers are legally required to use the same labeling as the brand-name drug. That means they can’t update warnings on their own-even if they know something’s wrong. The 2011 Supreme Court case PLIVA v. Mensing ruled that generic manufacturers can’t be sued for failing to warn. That created a reporting vacuum. When a patient has a bad reaction, the brand-name company often gets the report-even if the patient never took their drug. The FDA can’t tell which generic caused it.
That’s why your report matters. You know the exact manufacturer. You have the NDC code. You know the lot number. That data is gold.
What Happens When You Report?
Every report goes into the FDA’s FAERS database-over 25 million entries as of 2023. When enough reports point to the same issue, the FDA investigates. In 2022, 147 generic drugs were put under additional review because of pharmacist-submitted reports. Twelve of those led to direct warnings to prescribers and patients.
One case involved a generic version of levothyroxine. Pharmacists in several states noticed patients developing symptoms of hypothyroidism after switching to a new manufacturer. The reports piled up. The FDA reviewed the bioequivalence data, retested batches, and issued a safety communication. That’s how the system works.
But it only works if you report.
How to Report: A Simple Step-by-Step Guide
Reporting doesn’t have to be complicated. Here’s how to do it in under 10 minutes:
- Identify the issue. Was there a change in effectiveness, side effects, or tolerability after switching generics?
- Gather the details. Patient’s age (or initials if needed), drug name, manufacturer, NDC code, lot number, date of issue, and what happened.
- Go to the MedWatch portal. Visit www.fda.gov/medwatch and select Form 3500.
- Check “generic drug concern.” Choose the correct category: therapeutic inequivalence, manufacturing issue, or labeling error.
- Submit. You can report anonymously if you prefer. But including your name and contact info helps the FDA follow up if they need more info.
Pro tip: Save a copy of your report. Document it in the patient’s record. If another patient has the same issue, you’ll have proof you’ve flagged it.

State Rules Vary-Know Your Local Requirements
Federal law doesn’t force you to report. But 28 states have added their own rules. California, Illinois, Massachusetts, and New York require pharmacists to report serious adverse events. In California, the Board of Pharmacy explicitly says pharmacists must “maintain a system for identifying, documenting, and reporting adverse drug reactions.”
If you work in one of those states, you’re not just doing the right thing-you’re complying with the law. Even if you’re not, it’s still part of your professional duty. The American Society of Health-System Pharmacists says adverse event reporting is a “fundamental professional responsibility.”
The Bigger Picture: Why This Isn’t Just a Form
Generic drugs make up 90% of all prescriptions filled in the U.S. They’re essential for keeping healthcare affordable. But affordability shouldn’t mean lower safety.
When pharmacists don’t report, the FDA is flying blind. They rely on manufacturers to report problems-but manufacturers have a financial incentive to downplay issues. They’re not the ones seeing the patient come back with a rash, or the elderly woman who can’t walk after switching her statin.
Pharmacists are the eyes on the ground. Your reports help catch problems before they become nationwide crises. They help update labels, recall bad batches, and protect patients.
It’s not about blaming manufacturers. It’s about making sure every pill-no matter the brand-works as it should.
What You Can Do Today
Start small.
- Next time a patient says, “This generic doesn’t feel right,” ask: “When did you switch? Which brand?”
- Keep a log of any recurring issues with specific generics.
- Set a reminder: report one case a month. Even one report can make a difference.
- Share the MedWatch link with your team. Make it part of your safety culture.
You don’t need to be a superhero. You just need to be consistent.
Every report you submit is a piece of the puzzle. The FDA can’t fix what they don’t know. And right now, they’re missing a lot.



Geri Rogers
February 2, 2026This is SO important!! 🙌 I’ve seen patients break out in hives after switching generics-no one else connects the dots. I report every single time now. Even if it’s just ‘felt weird’-write it down. The FDA needs our eyes. 📊💊
Prajwal Manjunath Shanthappa
February 3, 2026Oh, please. You're suggesting that pharmacists-overworked, underpaid, and barely trained in pharmacovigilance-are somehow the arbiters of drug safety? The FDA has entire departments for this. You're indulging in a dangerous myth of professional omnipotence. 🤦♂️
Alex LaVey
February 5, 2026Hey, I just want to say-thank you for writing this. I’m a new pharmacist in rural Ohio, and honestly? I didn’t know I could report like this. I thought it was just for doctors or big hospitals. But now I get it: I’m the last person who sees the pill before it goes in. That’s powerful. I’m printing this out and putting it on the counter. 🙏
caroline hernandez
February 7, 2026Therapeutic inequivalence is a pharmacokinetic red flag that’s chronically underreported due to systemic workflow inefficiencies and regulatory ambiguity. The NDC code, lot number, and temporal correlation with symptom onset are critical data points for signal detection in FAERS. If you're not capturing this in your EHR with structured fields, you're missing a core component of pharmacovigilance. Mandatory reporting in 28 states isn't a suggestion-it's a clinical imperative. Start using templates. Automate. Your data saves lives.
Jhoantan Moreira
February 8, 2026Really appreciate this post. I’m from the UK and we don’t have the same generic system, but I’ve seen similar issues with biosimilars. The fact that pharmacists are the frontline here is both inspiring and heartbreaking. We need more systems that make reporting easy-like a one-click button in the pharmacy software. Keep doing the work, even if it’s quiet. It matters.
Joseph Cooksey
February 10, 2026Let’s be real-most of these ‘generic problems’ are just patients being dramatic or non-compliant. I’ve had the same guy come back every month saying ‘this generic made me tired’-but he’s on 12 meds, drinks 3 energy drinks a day, and hasn’t slept in a week. Meanwhile, the FDA gets flooded with nonsense reports from people who don’t understand bioequivalence. You don’t report a ‘feeling off’-you report a hospitalization. Otherwise, you’re just adding noise to a system already drowning in it. 🤷♂️
Justin Fauth
February 12, 2026They let some foreign company in China make our thyroid meds and now we’re supposed to just shrug and say ‘oh well’? This is why America’s healthcare is falling apart. I’ve got a cousin who went into seizures because the generic didn’t work-same dose, same pill, different manufacturer. And the FDA? They don’t even know which one it was. This is a national security issue. Someone needs to shut down these foreign labs. 🔥🇺🇸
Sherman Lee
February 13, 2026They’re watching you. The FDA, the pharma giants-they’re using your reports to build profiles. Every time you submit a MedWatch form, they tag your pharmacy, your patients, your IP. That’s how they know who’s ‘non-compliant’ or ‘high-risk.’ Next thing you know, your insurance gets flagged. This isn’t safety-it’s surveillance. And the ‘checkbox’? It’s a trap. Don’t fall for it. 🕵️♂️
Coy Huffman
February 15, 2026man i just started as a pharmacy tech and i had no idea any of this was a thing. i thought generics were just cheaper versions of the same thing. but now i see... it's like buying two different brands of cereal that say they're the same but one makes your stomach hurt. weird. i'm gonna start writing down the lot numbers now. thanks for the heads up 🤝
Amit Jain
February 16, 2026My friend in India says same thing. Generic medicine not work same. People get sick. Report it. Simple. No need big words. Just write down what happened. FDA will listen.
Keith Harris
February 17, 2026Oh wow, so now pharmacists are supposed to be drug cops? Next they’ll make us do the FDA’s job while we’re filling 80 scripts an hour. And who pays for the time? The patient? The pharmacy? No one. This is a classic case of corporate greed shifting responsibility onto the most vulnerable workers. Meanwhile, the brand-name companies who own the patents are laughing all the way to the bank. So let me get this straight-I’m supposed to risk my job to fix a system designed to fail? Yeah, right. 😏