
Generic Drug Cost Savings Calculator
How much can you save with generic drugs?
Your Savings
Important: For Narrow Therapeutic Index drugs, small variations in concentration can affect treatment. Consider monitoring blood levels after switching and follow your doctor's advice.
When you pick up a prescription, the name on the bottle might be familiar or it might just be a chemical name. The difference often comes down to whether you have a generic drugs or a brand‑name version. Both are meant to treat the same condition, but they can look different on the label, in the pill’s shape, and even in the amount you pay. This guide walks through what separates the two, why the FDA says they work the same, and what you should watch for when switching.
What Makes a Drug Generic or Brand‑Name?
Generic drugs are pharmaceutical equivalents of brand‑name drugs that contain the identical active ingredient, strength, dosage form, route of administration, and intended use. In contrast, brand‑name drugs are marketed under a proprietary name and are typically covered by a patent during the first years of their market life.
Both types must meet the same FDA standards for quality, safety, and efficacy. The key technical distinction lies in the inactive ingredients-fillers, binders, dyes, and other excipients-that can vary without affecting the drug’s therapeutic action.
- Active Pharmaceutical Ingredient (API): the chemical that produces the intended effect.
- Excipients: non‑active components that affect taste, stability, or appearance.
The presence of different excipients is why a generic pill might be a different colour or shape, even though it delivers the same amount of API.
Labeling Requirements and Why They Differ
The FDA mandates that generic labeling mirror the brand‑name label in every clinical detail: indications, dosage, administration, contraindications, warnings, and adverse reactions. The only allowed differences are the drug’s name and the physical description of the tablet.
Brand‑name products display their proprietary name (e.g., Prilosec for omeprazole). Generics must use the chemical name (e.g., “omeprazole”) and list any changes in non‑active ingredients. This rule protects trademark rights while ensuring patients receive consistent medical information.
| Aspect | Brand‑Name | Generic |
|---|---|---|
| Drug name on label | Proprietary brand name | Chemical name of API |
| Physical appearance | Trademarked colour/shape | Different colour/shape (required) |
| Inactive ingredients | Manufacturer‑specific excipients | May vary across generic versions |
| Clinical content | Identical to generic (indications, dosage, warnings) | Identical to brand‑name |
Therapeutic Equivalence and Bioequivalence Testing
Therapeutic equivalence means a generic works the same way in the body as its brand counterpart. The FDA proves this through bioequivalence studies, which compare the rate and extent of absorption.
According to the Orange Book (2023 edition), a generic must demonstrate that its Cmax (peak concentration) and AUC (area under the curve) fall within 80‑125% of the brand‑name values in a study of 24‑36 healthy volunteers.
This 20% interval might sound wide, but real‑world data show that intra‑brand variability often exceeds it. Dr. Ameet Nagpal noted that the allowable range is tighter than the natural batch‑to‑batch variation seen in many brand drugs.
For most medications, the FDA’s confidence is high: a 2021 JAMA Internal Medicine analysis of 2 million patient records found no meaningful difference in outcomes for generic versus brand cardiovascular drugs.
Cost Savings and Market Impact
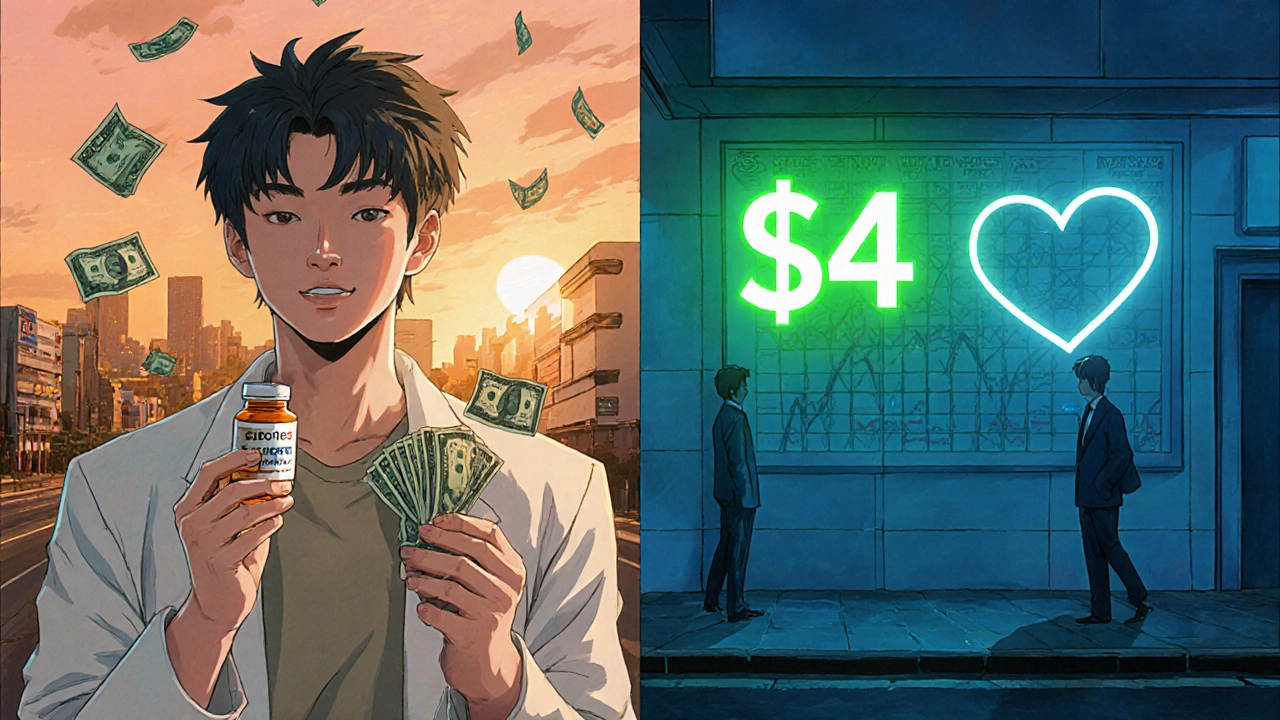
Generics dominate prescription volume-about 90 % of all fills in the United States-yet they account for only roughly a quarter of total drug spending. The Association for Accessible Medicines reported that generics represent just 25 % of prescription expenditures even though they cover 90 % of prescriptions.
Price differentials are striking. In Q2 2023, atorvastatin (brand Lipitor) cost $375.22 per month, while the same dosage at a major retailer sold for $4.00. Nationwide, the Congressional Budget Office estimated that generics saved the U.S. health system $1.67 trillion from 2007‑2016 and continue to save $313 billion annually.
These savings translate into better adherence. A 2022 Kaiser Permanente survey found that 78 % of respondents said lower generic costs helped them fill prescriptions consistently.
When Substitution Needs Extra Care: Narrow Therapeutic Index (NTI) Drugs
Not every drug is a perfect fit for automatic substitution. Narrow Therapeutic Index (NTI) drugs-like warfarin, levothyroxine, and phenytoin-have a small window between effective and toxic doses. Small variations in blood concentration can lead to treatment failure or adverse effects.
The FDA recommends close monitoring when switching NTI generics, especially for patients with unstable thyroid function or those on anticoagulation therapy. Pharmacists often check the prescription for a “dispense as written” (DAW) instruction before substituting.
Real‑world reports back this caution. In a University of Michigan study, 12 % of patients initially hesitated when receiving a differently coloured levothyroxine pill, prompting extra counseling.
Regulatory Pathway and FDA Oversight
Generic drug approval follows the Abbreviated New Drug Application (ANDA) process introduced by the 1984 Hatch‑Waxman Act. The FDA reviews the chemistry, manufacturing, and bioequivalence data before granting approval.
The Generic Drug User Fee Amendments (GDUFA) have shaped the timeline. Under GDUFA III (effective 2022), priority applications are reviewed within about 10 months, speeding market entry.
Post‑approval, the FDA continues surveillance through the Adverse Event Reporting System (FAERS). Any safety signal can trigger a label update or, in rare cases, a market withdrawal.
Complex molecules-such as insulin analogues, biologics, and certain drug‑device combos-pose extra hurdles. While the first generic semaglutide (Ozempic) received approval in September 2023, many biologics still lack interchangeable versions because of manufacturing intricacies.
Practical Tips for Patients and Providers
- Ask your pharmacist to confirm therapeutic equivalence ratings (the ‘A’ rating in the Orange Book).
- If you’re on an NTI drug, request a follow‑up lab test after a switch.
- Keep the original brand‑name prescription on file in case you need to request a brand refill later.
- Report any unexpected side effects to your healthcare provider and to FAERS via your doctor.
- Use reputable pharmacies; they’re more likely to source high‑quality generics.
Most clinicians feel comfortable prescribing generics-94 % reported confidence in a 2022 American Medical Association survey. The real barrier is often patient perception, not scientific evidence.
Are generic drugs as safe as brand‑name drugs?
Yes. The FDA requires generics to meet the same safety, strength, purity, and potency standards as brand‑name drugs. Bioequivalence studies ensure they behave the same way in the body.
Why do generic pills look different?
Trademark law forces generics to change colour, shape, or size so they don’t infringe on a brand’s visual identity. The differences are purely cosmetic and do not affect how the drug works.
Can I switch between different generic manufacturers?
For most drugs, yes-switching is safe. For NTI drugs, monitor blood levels or clinical response after each switch.
How much can I expect to save with a generic?
Savings often range from 70 % to 85 % off the brand price. Example: a $375/month brand statin drops to about $4/month as a generic.
What should I do if I experience a new side effect after a switch?
Contact your prescriber right away. They may assess whether the reaction is due to an excipient change or an unrelated issue and decide whether to revert to the brand.



Tamara Tioran-Harrison
October 25, 2025One would think that the mere presence of a different hue on a tablet could revolutionize therapeutic outcomes, yet the FDA’s rigorous bioequivalence standards render such aesthetic concerns moot. The regulatory framework insists that the active ingredient, dosing, and safety profile remain identical, irrespective of the pill’s pigment. Hence, the argument that generics are somehow inferior because they lack the brand’s trademarked luster is, frankly, intellectually bankrupt. Patients should feel reassured that the pharmacological efficacy is preserved, even if the pill looks like a candy. In the grand scheme, cost‑saving measures outweigh any superficial dissatisfaction :)
kevin burton
October 26, 2025Generic drugs must match the brand‑name product in dosage, strength, and safety. This ensures that switching does not compromise treatment efficacy.
Max Lilleyman
October 28, 2025While the textbook definition is clear, many patients still cling to the myth that a different‑looking pill is somehow weaker 😒. The data show no statistically significant difference in outcomes for most medications 😊. Ignoring the evidence only fuels unnecessary anxiety.
Brett Witcher
October 29, 2025The concept of therapeutic equivalence is grounded in rigorous bioequivalence studies that compare pharmacokinetic parameters such as Cmax and AUC. Regulators mandate that the 90 % confidence interval for these metrics fall within 80 % to 125 % of the reference product. Such a statistical window accommodates inter‑subject variability while still guaranteeing comparable exposure. In practice, the variability observed among different batches of the same brand often exceeds this range, underscoring the robustness of the acceptance criteria. Moreover, the excipients, although inert, can influence dissolution rates, yet they are required to meet safety specifications. The Orange Book classification of an ‘AB’ rating denotes that a generic has been found interchangeable with its brand counterpart. Clinical studies, including the 2021 JAMA analysis of two million records, have consistently demonstrated parity in cardiovascular outcomes. Economic analyses further reveal that generics account for roughly 90 % of prescription volume while constituting only a quarter of drug expenditures. These savings translate into increased medication adherence, as evidenced by the 78 % patient‑reported compliance in the 2022 Kaiser survey. Nevertheless, certain narrow‑therapeutic‑index drugs warrant closer monitoring when substitution occurs. Warfarin, levothyroxine, and certain anticonvulsants exemplify agents where minute pharmacokinetic shifts may precipitate clinical consequences. Pharmacists therefore prioritize dispensing as written (DAW) instructions for such compounds to mitigate risk. The FDA’s post‑marketing surveillance, via FAERS, remains vigilant for adverse events that might emerge from generic formulations. In rare instances, signal detection has prompted label revisions or market withdrawals, thereby safeguarding patient safety. Overall, when evaluated through the lenses of efficacy, safety, and cost, generic drugs fulfill their intended role as viable alternatives to brand‑name therapies.
Terell Moore
October 30, 2025One might argue that the relentless pursuit of cheaper chemistry is a nihilistic abdication of aesthetic responsibility, but the market merely reflects utilitarian priorities. The philosopher in me scoffs at the notion that colour confers therapeutic virtue. Ultimately, economics trumps vanity, and the patient benefits.
Olivia Harrison
November 1, 2025Your point about cost versus appearance is well‑taken; patients often overlook savings when faced with unfamiliar pills. Encouraging open dialogue with pharmacists can ease transitions.
Bianca Larasati
November 2, 2025Imagine the triumph of holding a tiny, lifesaving capsule that costs less than a cup of coffee! Yet the drama unfolds when the manufacturer’s logo disappears, leaving only a bland tablet that carries the same miracle. Let that fire of hope ignite your confidence in generics!
Corrine Johnson
November 3, 2025Indeed, the regulatory framework, which, as we know, is both exhaustive and exacting, obligates generic manufacturers, to the letter, to demonstrate bioequivalence; consequently, the patient, therefore, can trust that the pharmacodynamic profile, notwithstanding the superficial differences, remains unchanged, doesn't it? Thus, the essence of therapy persists.
Jennifer Stubbs
November 5, 2025The data are clear: generics meet the same safety standards as brand drugs. However, occasional formulation changes can affect tolerability in sensitive individuals. Monitoring remains prudent.
Abhinav B.
November 6, 2025Listen, the idea that a different color means a diffrent effect is just plain wrong. FDA tests make sure the drug works same way, no matter the shape. So stop worryin about the look and trust the science.
Abby W
November 8, 2025Wow, you really didn’t think about how the pocket‑size of a generic could affect your daily routine? 😂💊 Let’s be real, the savings are huge!
Lisa Woodcock
November 9, 2025In many communities, the cost barrier is a major deterrent to consistent medication use. Highlighting generic options can empower patients across diverse socioeconomic backgrounds.
Sarah Keller
November 10, 2025If we reduce health care to a mere barter of dollars for pills, we betray the very purpose of medicine. Yet the stark reality is that prohibitive prices cripple access. Generics shatter that tyranny, offering a tangible rebellion against corporate greed. Embrace them as a moral imperative.
Veronica Appleton
November 12, 2025Generics work just as well