When you take digoxin, even a tiny change in how much of the drug gets into your bloodstream can mean the difference between healing and hospitalization. That’s because digoxin is a narrow therapeutic index drug - a class where the gap between a safe dose and a toxic one is razor-thin. The therapeutic range? Just 0.5 to 2.0 ng/mL. Go below that, and heart failure symptoms creep back. Go above, and you risk dangerous arrhythmias, vomiting, blurred vision, or worse.
Why Generic Digoxin Isn’t Like Other Generics
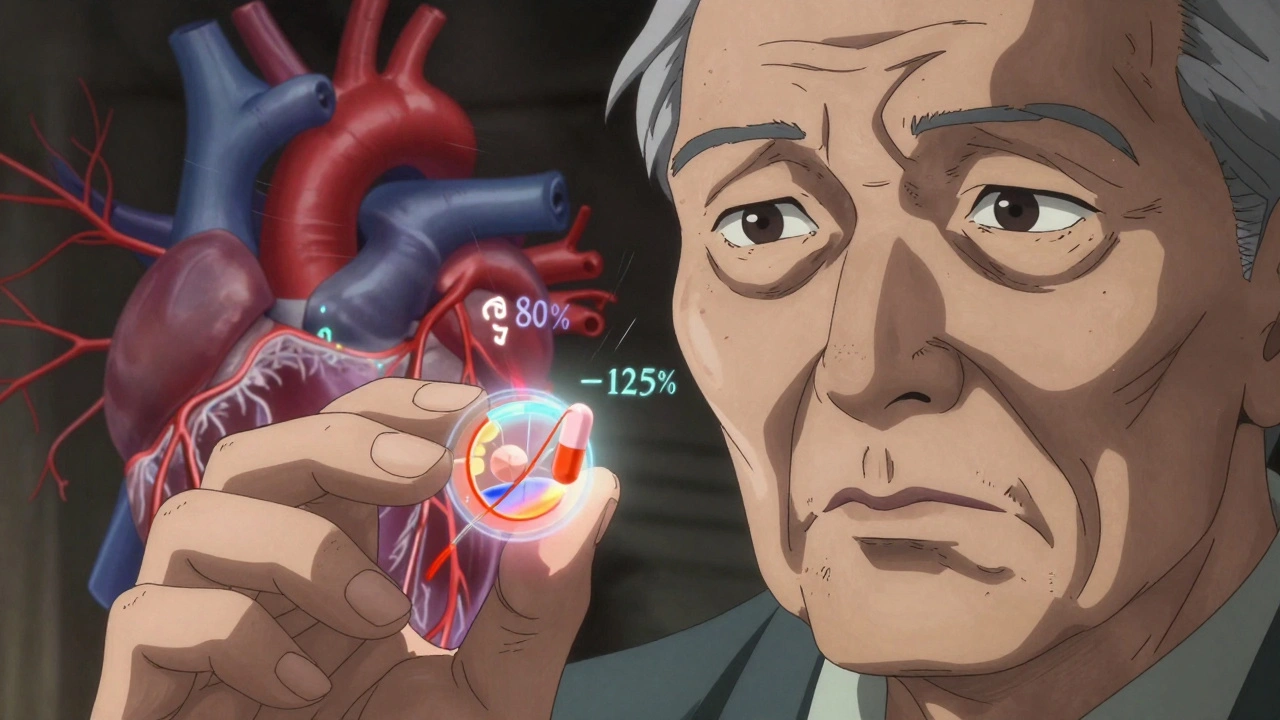
Most generic drugs are straightforward swaps. You switch from brand to generic, and your body barely notices. But digoxin? It’s different. The FDA treats it like a new drug, not just a copy. Why? Because small differences in how the body absorbs it - called bioavailability - can have life-or-death consequences.For most medications, bioequivalence means the generic must deliver 80-125% of the active ingredient compared to the brand (Lanoxin). That’s fine for drugs with wide safety margins. But for digoxin, even a 10% drop in absorption can push levels below the therapeutic range. A 10% spike? That might land you in the ICU.
The FDA requires every generic digoxin tablet to prove it matches Lanoxin’s absorption profile using strict pharmacokinetic studies. They measure two key numbers: AUC (how much drug enters the blood over time) and Cmax (the highest concentration reached). The 90% confidence interval for both must fall between 80% and 125% of Lanoxin’s values. That sounds tight - and it is. Only three generic digoxin tablets currently carry an "AB" rating in the FDA’s Orange Book, meaning they’ve passed this test.
The Hidden Problem: Switching Between Generics
Here’s where things get risky. Just because Generic A is bioequivalent to Lanoxin, and Generic B is also bioequivalent to Lanoxin, doesn’t mean Generic A and Generic B are bioequivalent to each other. There are no studies proving that.Imagine you’ve been stable on Generic A for six months. Your heart feels better. Your digoxin level is 0.8 ng/mL - right in the sweet spot. Then your pharmacy switches you to Generic B because it’s cheaper. You don’t notice anything different at first. But three days later, your pulse is irregular. You feel nauseous. A blood test shows your digoxin level is now 2.4 ng/mL - toxic.
That’s not hypothetical. Real cases like this have been documented. One study found that switching between generic digoxin brands caused serum concentration changes of over 25% in some patients. That’s enough to trigger toxicity in someone already on the edge.
This isn’t about quality control. It’s about formulation. Different manufacturers use different fillers, binders, and coatings. These ingredients affect how fast the tablet dissolves in your gut. For digoxin, even a few minutes’ delay in dissolution can change absorption enough to matter.
Formulation Matters: Tablets vs. Elixir
Not all digoxin forms are created equal. The tablet version has variable absorption - typically 60-80% of the intravenous dose. But the oral elixir? It’s absorbed much better: 70-85%. That’s why some patients, especially those with swallowing issues or poor gut motility, are prescribed the liquid form. But switching from tablet to elixir - or vice versa - without adjusting the dose can be dangerous.One patient on 0.125 mg tablets daily might need only 0.1 mg of elixir to get the same effect. But if you just swap them without checking levels, you’re playing Russian roulette with their heart rhythm.
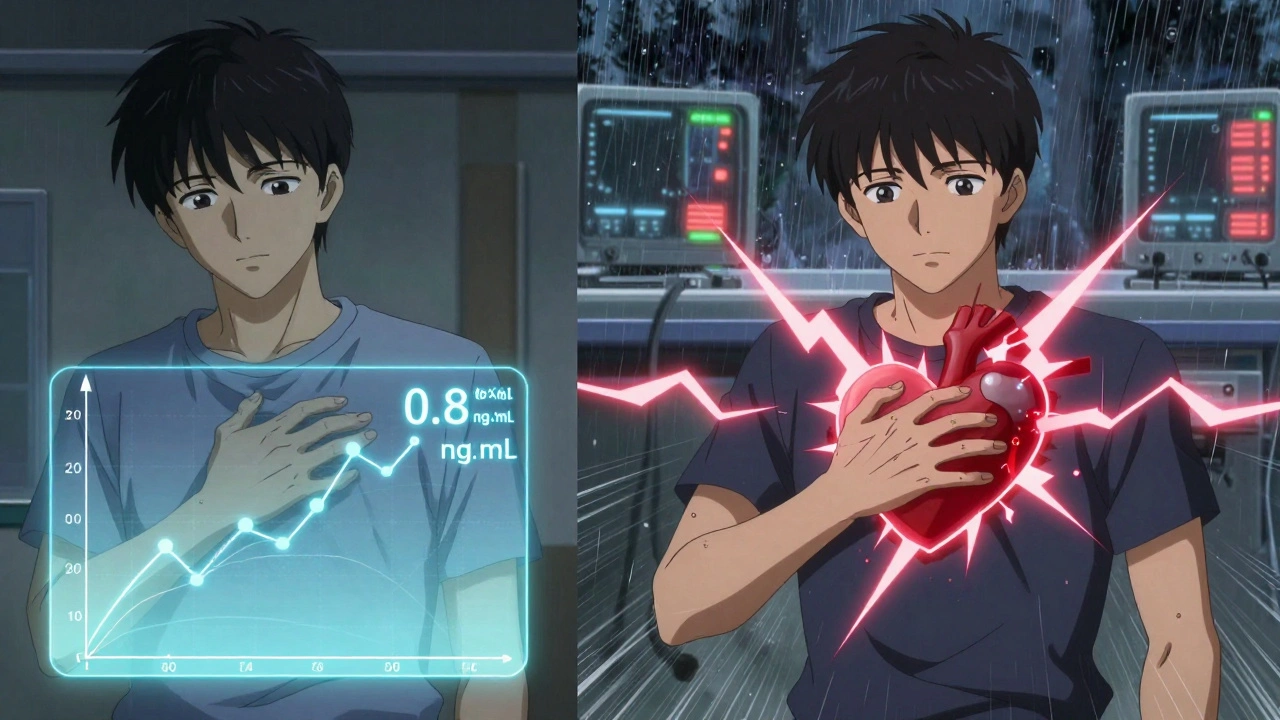
Who’s Most at Risk?
The majority of digoxin users are older adults - often over 70. Many have reduced kidney function, which slows how fast the drug leaves the body. That means digoxin builds up over time. Add to that: multiple medications, changing diets, dehydration, or even a bout of the flu - and the risk of toxicity climbs.Patients with atrial fibrillation or heart failure are especially vulnerable. Their condition depends on precise digoxin levels to control heart rate and improve pumping. Too little? Their heart races again. Too much? They develop a dangerous, irregular rhythm called ventricular tachycardia.
Studies show that for heart failure patients, keeping digoxin levels between 0.5 and 0.9 ng/mL reduces death risk. Higher levels don’t help - they hurt. That’s why the American College of Clinical Pharmacy recommends targeting the lower end of the therapeutic range, not the middle.
When and How to Monitor
You can’t guess digoxin levels. You can’t rely on symptoms alone. Toxicity can sneak up slowly. That’s why regular blood tests aren’t optional - they’re essential.Here’s what you need to know:
- Check serum levels 4-7 days after starting digoxin or changing the dose. It takes about a week for levels to stabilize.
- Always draw the blood just before the next dose - that’s the "trough" level, and it’s the most accurate.
- Test again after switching manufacturers, changing kidney function, adding or stopping other drugs (like antibiotics or diuretics), or if symptoms change.
- If you switch from one generic to another, check levels 3-5 days after the change. Don’t wait for symptoms.
Some doctors skip these checks because they assume "all generics are the same." But with digoxin, that assumption kills. The American Heart Association and American College of Cardiology both say: use the same manufacturer’s product whenever possible. If you must switch, monitor.
What Clinicians Should Do
If you’re prescribing digoxin, here’s your action plan:- Start with the brand-name Lanoxin if possible - especially for new patients.
- If using generics, stick to one manufacturer. Document the brand name and lot number on the prescription.
- Never switch generics without checking levels. Treat digoxin like insulin or warfarin - not like aspirin.
- Teach patients to recognize early signs of toxicity: nausea, loss of appetite, yellow-green halos around lights, dizziness, or irregular heartbeat.
- Consider lower targets: 0.5-0.9 ng/mL for heart failure. Higher levels offer no extra benefit and increase risk.
Pharmacists play a role too. If a patient is on digoxin and a generic switch is proposed, flag it. Call the prescriber. Ask: "Have levels been checked since the last change?"
The Bottom Line
Generic digoxin is not unsafe. Many are bioequivalent to Lanoxin. But bioequivalence at the population level doesn’t guarantee safety for every individual. The system works on averages. But digoxin doesn’t care about averages - it cares about your body, your kidneys, your other meds, and the exact tablet you swallowed this morning.The data is clear: switching between generic digoxin products without monitoring can cause dangerous fluctuations in blood levels. That’s why experts agree - consistency matters more than cost savings.
For patients on digoxin, the safest approach isn’t the cheapest one. It’s the one where levels are checked, changes are tracked, and the same product is used - month after month, year after year.

Chris Wallace
December 3, 2025Man, I’ve seen this play out with my grandma. She was on digoxin for years, stable as a rock on the same generic brand. Then the pharmacy switched her to a cheaper one ‘cause of insurance. No warning, no lab check. Three days later she’s in the ER with visual halos and a heart rate that looked like a seizure on the monitor. They had to reverse it with digibind. She’s fine now, but it scared the hell out of us. I didn’t even know generics could be this unpredictable. Now I make sure her script says the exact brand name. It’s not about cost-it’s about not losing someone over a tablet coating.
Doctors act like it’s aspirin. It’s not. It’s more like insulin with a mood swing.
william tao
December 4, 2025The FDA’s bioequivalence standards for digoxin are laughably inadequate. Eighty to one hundred twenty-five percent? That’s a 45% window for variability. In any other therapeutic context, this would be considered criminal negligence. We are allowing pharmaceutical companies to manufacture life-threatening inconsistencies under the guise of ‘cost-effective healthcare.’ This is not pharmacology-it’s roulette with a cardiac arrest jackpot.
And yet, the system continues. Because profit > patient.
Sandi Allen
December 5, 2025Who’s really behind this? The big pharma lobby, of course. They push generics so they can keep selling the brand-name version to the rich while dumping the ‘almost-the-same’ crap on Medicare patients. And don’t get me started on the ‘AB’ rating-what a joke! It’s not a rating, it’s a trap. The FDA is complicit. They don’t test real-world switching scenarios. They test lab rats in controlled environments. Real people? We’re the experiment.
I’ve seen patients die from this. And no one’s getting sued. No one’s going to jail. Just more paperwork. More silence. More bodies.
Call your rep. Demand a ban on generic digoxin switches. Or your mother’s next ‘heart episode’ might be her last.
Shubham Pandey
December 6, 2025Generic digoxin bad. Stick to one brand. Check levels. Done.
Genesis Rubi
December 6, 2025Why are we even letting these foreign-made generics in? I mean, come on. China and India are pumping out pills in factories where the air smells like rust and regret. How do we know what’s really in them? One of those ‘bioequivalent’ tablets could be 70% filler and 30% wishful thinking. We’re trusting our grandparents’ hearts to overseas quality control? No thanks. I’d rather pay $20 more for Lanoxin than bury someone because of a cheap tablet.
Also, why is the FDA even allowing this? We’re the United States of America. We don’t settle for ‘close enough.’
Doug Hawk
December 7, 2025What’s fascinating here is the pharmacokinetic disconnect between population bioequivalence and individual pharmacodynamics. The 80-125% AUC/Cmax range is statistically acceptable for drugs with wide TI, but digoxin’s TI is 4:1. That’s not a margin-it’s a chasm. The FDA’s regulatory framework was built for ibuprofen, not cardiac glycosides. We’re applying population-level statistics to a drug where inter-individual variability is the primary risk factor.
And yet, we still don’t mandate therapeutic drug monitoring for every switch. Why? Because it’s inconvenient. Because labs cost money. Because physicians are overworked. Because we’ve normalized risk in chronic care.
It’s not negligence. It’s systemic failure dressed up as efficiency.
John Morrow
December 8, 2025Let’s be brutally honest: most prescribers don’t understand digoxin. They think it’s ‘just another heart pill.’ They don’t know the difference between tablet and elixir absorption. They don’t know that a 0.1 mg elixir isn’t equivalent to 0.125 mg tablet. They don’t know that renal clearance drops 40% after age 70. And they certainly don’t check levels unless the patient’s already coding.
This isn’t a drug issue. It’s a knowledge gap issue. And until medical schools stop teaching digoxin like it’s a relic from the 1970s-instead of a precision instrument-we’ll keep seeing these preventable deaths.
And yes, I’ve seen it. Multiple times. The EKG looks like a seizure. The patient smells like rotten apples. And the chart says ‘generic digoxin 0.125 mg daily.’ No notes. No history. No monitoring.
It’s not a miracle drug. It’s a landmine.
Kristen Yates
December 8, 2025I work in a rural clinic. Our patients are mostly elderly, on fixed incomes. They switch pharmacies all the time. Sometimes they don’t even know they’re on a different generic. I’ve had to call pharmacies three times just to confirm what exact tablet they’re taking. I write the brand name on every script now-even if it’s generic. I tell them: ‘This isn’t like switching from Coke to Pepsi. This is like switching from water to poison.’
One woman cried when I told her we’d need to check her levels. She said, ‘I just want to feel okay.’ I told her I know. And we’ll make sure you do. One pill. One level. One month at a time.
Saurabh Tiwari
December 9, 2025Bro this is wild but makes total sense 🤯
in india we have like 10 different digoxin brands and no one checks levels 😅
but honestly most patients here are too poor to even get regular blood tests so they just keep taking whatever’s cheapest
maybe we need a global standard? or at least color coded pills or something?
like red for high risk switch, green for safe brand
just a thought 🤔
Michael Campbell
December 10, 2025They’re lying. All of them. The FDA, the pharmacists, the doctors. They know this kills people. But they don’t care. Because the insurance companies won’t pay for Lanoxin. Because the pharmacy chains make more profit off generics. Because no one’s gonna sue a $2 pill.
I lost my uncle to this. They switched him to a new generic. Said it was ‘the same.’ He died two weeks later. Autopsy said digoxin toxicity. No one checked his levels. No one asked what brand he was on.
It’s murder by bureaucracy.
Victoria Graci
December 10, 2025There’s something almost poetic about digoxin. It’s a plant-derived compound, once used by medieval healers to treat dropsy. Now it’s a chemical chess game played in hospital labs with blood draws and pharmacokinetic curves. We’ve turned an ancient remedy into a high-stakes algorithm-but we still treat it like a commodity.
It’s not just about absorption rates or tablet coatings. It’s about how we value human life in a system that measures worth in cost-per-dose. We optimize for efficiency, but digoxin doesn’t optimize. It remembers. It accumulates. It waits.
Maybe the real question isn’t ‘why is this dangerous?’
It’s ‘why do we keep pretending it isn’t?’