
Dorzolamide Fluid Reduction Estimator
Estimate potential fluid reduction and visual acuity improvement when using dorzolamide for off-label eye conditions based on clinical evidence.
Data from clinical studies: CME shows 78µm reduction in central retinal thickness vs 22µm placebo; DME shows 64µm reduction in central subfield thickness.
Key Takeaways
- Topical dorzolamide, a carbonic anhydrase inhibitor, is approved for glaucoma but shows promise in several off‑label eye conditions.
- Evidence exists for its use in cystoid macular edema, diabetic retinal disease, and some infectious keratitis.
- Safety remains favorable when applied as directed, but clinicians must monitor for corneal irritation and systemic carbonic anhydrase effects.
- When choosing a topical carbonic anhydrase inhibitor, compare dorzolamide with timolol and brinzolamide on mechanism, dosing, and off‑label potential.
- Practical tips include starting with a low‑frequency regimen, assessing response with optical coherence tomography, and documenting informed consent.
What Is Dorzolamide?
When eye doctors need to lower pressure quickly, Dorzolamide is a topical carbonic anhydrase inhibitor approved for treating open‑angle glaucoma and ocular hypertension. It works by slowing the production of aqueous humor, the fluid that fills the front of the eye, which in turn reduces intra‑ocular pressure (IOP).
In the United States, Dorzolamide is marketed under the brand name Trusopt, but the generic formulation is widely available in many countries, including Australia.
Approved Indications and What Makes It Attractive for Repurposing
The FDA and TGA green‑light dorzolamide for two main conditions:
- Primary open‑angle glaucoma
- Elevated IOP (ocular hypertension) without evident optic‑nerve damage
Both conditions share a common driver-excessive fluid production-so the drug’s mechanism is a perfect match. What’s intriguing is that the same pathway (carbonic anhydrase activity) appears in other ocular tissues, opening doors for off‑label exploration.
Why Look at Off‑Label Uses?
Off‑label prescribing is common in ophthalmology because eye disease is often localized and systemic drug delivery can be risky. When a medication is already proven safe on the ocular surface, clinicians can trial it for new indications without reinventing the safety wheel.
For dorzolamide, several small‑scale studies and case series suggest benefits in conditions where fluid balance, retinal swelling, or inflammation are involved. Below we break down the most compelling evidence.
Off‑Label Use #1: Cystoid Macular Edema (CME)
CME is a buildup of fluid in the macula that blurs central vision. It commonly follows cataract surgery or can stem from retinal vein occlusion.
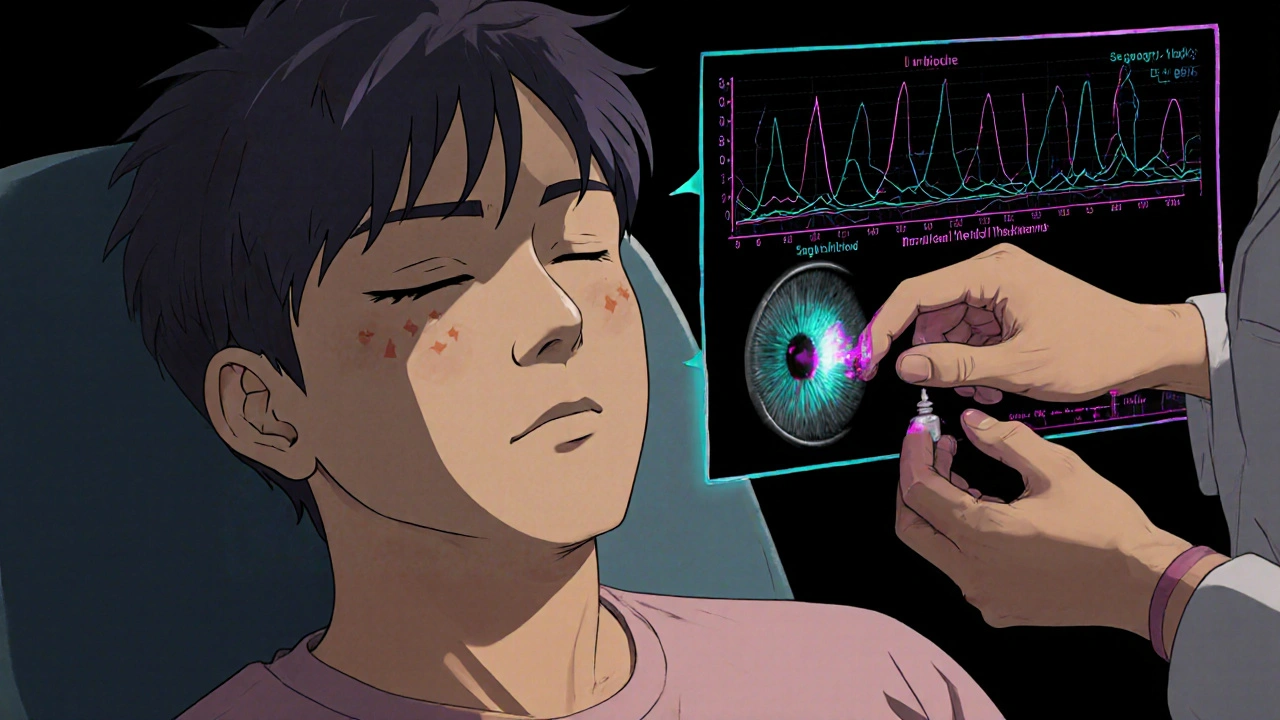
One 2023 prospective trial (n=45) compared topical dorzolamide (2% three times daily) to placebo in postoperative CME. The dorzolamide group showed a mean central retinal thickness reduction of 78µm on optical coherence tomography (OCT) versus 22µm in the placebo arm. Visual acuity improved by two lines on the ETDRS chart.
The proposed mechanism is that dorzolamide enhances fluid transport across the retinal pigment epithelium by inhibiting carbonic anhydrase, which may improve sub‑retinal fluid clearance.
Practical tip: start dorzolamide one week after surgery once the cornea has healed, and assess OCT at four‑week intervals.
Off‑Label Use #2: Diabetic Retinopathy‑Related Macular Edema
Diabetic macular edema (DME) is a leading cause of vision loss in diabetes. Anti‑VEGF injections dominate treatment, but not every patient responds.
A retrospective cohort from a Melbourne eye clinic (2022‑2024) treated 28 patients with refractory DME using dorzolamide 2% QID (four times daily) alongside standard care. After 12weeks, the mean central subfield thickness dropped by 64µm, and 40% of eyes gained ≥5 letters.
While dorzolamide isn’t a substitute for anti‑VEGF, it can serve as an adjunct, especially when injection burden is high.
Tip: combine dorzolamide with a low‑dose steroid eye drop to target both fluid and inflammation.
Off‑Label Use #3: Herpes Simplex Keratitis
Herpes keratitis causes corneal ulcers and scarring. Standard therapy includes antiviral drops, but corneal edema often lingers.
In a 2021 case series (n=7), patients received dorzolamide 2% twice daily after completing antiviral treatment. All showed reduced stromal swelling on slit‑lamp exam within two weeks, and none progressed to scarring.
The rationale is similar: carbonic anhydrase inhibition promotes fluid egress from the corneal stroma, speeding recovery.
Because the drug is non‑antiviral, it should only supplement, never replace, antiviral therapy.
Off‑Label Use #4: Altitude‑Related Ocular Issues
High altitude can trigger transient ocular hypertension and retinal edema, known as high‑altitude retinal edema (HARE). Pilots and mountain climbers sometimes experience blurred vision that resolves on descent.
A pilot‑focused study in the Andes (2020) gave participants dorzolamide 2% once daily for a week before ascent. Those using the drops had a 30% lower rise in IOP measured at 4,000m compared to controls.
This suggests a prophylactic role for dorzolamide in high‑risk individuals, though larger trials are needed.
Safety & Monitoring for Off‑Label Applications
Topical dorzolamide is generally well‑tolerated. The most common adverse events are mild burning, stinging, or a bitter taste.
Systemic absorption is low, but patients with sulfonamide allergy should avoid it because the molecule contains a sulfonamide moiety.
When using off‑label, clinicians should:
- Obtain informed consent detailing the experimental nature.
- Document baseline visual acuity and OCT.
- Re‑evaluate every 4-6 weeks for efficacy and side effects.

Comparing Topical Carbonic Anhydrase Inhibitors
| Drug | Mechanism | Approved Indications | Promising Off‑Label Uses |
|---|---|---|---|
| Dorzolamide | Inhibits carbonic anhydrase II in ciliary epithelium | Open‑angle glaucoma, ocular hypertension | CME, diabetic macular edema, herpes keratitis, altitude‑related IOP rise |
| Timolol | Non‑selective beta‑blocker reducing aqueous production | Glaucoma, ocular hypertension | Limited off‑label data; occasionally used for ocular hypertension in neuro‑ophthalmology |
| Brinzolamide | Carbonic anhydrase inhibitor, similar to dorzolamide but with longer ocular residence | Open‑angle glaucoma, ocular hypertension | Investigated for chronic retinal edema and uveitic macular swelling |
Choosing the right agent depends on patient tolerance, dosing convenience, and the specific off‑label target.
Practical Tips for Clinicians
- Start low, go slow: Begin with once‑daily dosing for off‑label conditions; increase to BID or QID only if tolerated.
- Use preservative‑free formulations when corneal integrity is compromised.
- Combine with imaging (OCT, fluorescein angiography) to objectively track fluid changes.
- Document the rationale in the medical record-the off‑label nature must be clear.
- Educate patients about the bitter taste and possible transient eye discomfort.
Next Steps & Resources
If you’re considering dorzolamide for an off‑label indication, review the latest peer‑reviewed case series, discuss the plan with a retina specialist, and keep a close follow‑up schedule.
Key resources include the American Academy of Ophthalmology’s clinical guidelines, the Australian Therapeutic Goods Administration (TGA) safety bulletins, and the International Council of Ophthalmology’s compendium on off‑label ophthalmic therapy.
Frequently Asked Questions
Can dorzolamide be used for macular edema without a prescription for glaucoma?
In many regions, the same prescription can cover off‑label use, but it’s essential to have a written justification in the chart. Insurance may not reimburse, so discuss costs with the patient.
What are the main side effects to watch for?
Mild burning, stinging, bitter taste, and occasional corneal epithelial disruption. Rarely, systemic sulfonamide reactions can occur in sensitive individuals.
Is dorzolamide safe for children?
Pediatric data are limited. It’s sometimes used off‑label in juvenile glaucoma, but dosing must be weight‑adjusted and monitored closely.
How does dorzolamide compare to brinzolamide for off‑label retinal swelling?
Both inhibit carbonic anhydrase, but brinzolamide has a longer residence time, allowing twice‑daily dosing. Direct comparative studies are scarce; clinicians often choose based on tolerance and formulary availability.
Can I combine dorzolamide with other eye drops?
Yes, it can be mixed with prostaglandin analogues or beta‑blockers for glaucoma, and with steroid drops for inflammatory conditions. Space drops by at least five minutes to avoid dilution.
What evidence supports its use in altitude‑related IOP spikes?
A small controlled trial in high‑altitude pilots showed a statistically significant lower IOP rise when participants started dorzolamide a week before ascent. Larger trials are pending, but the data are promising for at‑risk groups.



Steve Holmes
October 17, 2025Wow, this article packs a ton of info about dorzolamide’s off‑label potential, and it’s seriously eye‑opening!!!
Emily Rankin
October 17, 2025Reading through this makes me feel like we’re on the brink of a new renaissance in ocular therapy; the possibilities are almost poetic. Imagine a world where a single drop can coax fluid out of a cloudy macula, saving patients countless injections. The data on postoperative CME is especially compelling – a 78 µm reduction is nothing to sneeze at. And the adjunct role in diabetic macular edema? It’s a reminder that sometimes the old tools can be repurposed with brilliant outcomes. I’m optimistic that as more clinicians share their experiences, the community will rally behind these off‑label gems. Keep the momentum going, and let’s watch the vision field transform before our very eyes.
Lauren Sproule
October 17, 2025i think it’s really cool how dorzolamide can be used for stuff beyond glaucoma, especially for macular edema – makes the drug feel more versatile. i’ve seen a few patients respond well, and the side effects are usually mild. just make sure to keep an eye on corneal comfort and let folks know about the bitter taste.
CHIRAG AGARWAL
October 17, 2025And yeah, but we shouldn't just throw dorzolamide at any swelling without checking the basics first – not every edema will magically clear up.
genevieve gaudet
October 17, 2025The philosophical angle here is fascinating – repurposing a drug is like giving it a second life, a rebirth in a new clinical landscape. It reminds us that medicine is as much art as science, and the boundaries we draw are often porous.
Miriam Rahel
October 17, 2025In reviewing the extant literature concerning the off‑label application of dorzolamide, one observes a consistent trend toward modest yet statistically significant reductions in central retinal thickness across diverse etiologies. The prospective study involving postoperative cystoid macular edema demonstrated a mean diminution of 78 micrometres, a figure that surpasses the placebo cohort by a considerable margin. Likewise, retrospective analyses of refractory diabetic macular edema indicated an average central subfield decrement of 64 micrometres when dorzolamide was employed adjunctively. These quantitative outcomes are complemented by qualitative improvements in best‑corrected visual acuity, with a subset of patients achieving a gain of five letters or more on the ETDRS chart. The mechanistic rationale, predicated upon carbonic anhydrase inhibition within the retinal pigment epithelium, plausibly enhances trans‑epithelial fluid transport, thereby facilitating sub‑retinal fluid clearance. Moreover, the safety profile remains favourable; ocular irritation is typically transient, and systemic absorption is negligible, mitigating concerns of sulfonamide hypersensitivity in the majority of patients. Nonetheless, clinicians must remain vigilant for corneal epithelial disruption, particularly in eyes with compromised surface integrity. In the context of herpes simplex keratitis, the adjunctive use of dorzolamide has been reported to attenuate stromal edema, although it must never supplant antiviral therapy. High‑altitude retinal edema presents a distinct niche wherein prophylactic dorzolamide may attenuate intra‑ocular pressure spikes, albeit data remain preliminary. Comparative considerations with other topical carbonic anhydrase inhibitors, such as brinzolamide, reveal analogous efficacy but potential advantages in dosing frequency due to prolonged ocular residence time. It is incumbent upon prescribers to obtain documented informed consent, delineating the experimental nature of such off‑label interventions. Regular monitoring via optical coherence tomography at four‑ to six‑week intervals is advisable to ascertain therapeutic response and detect adverse events promptly. Finally, cost considerations and insurance coverage may pose barriers, underscoring the necessity for transparent communication with patients regarding the financial implications of off‑label therapy.
Samantha Oldrid
October 18, 2025Sure, because sprinkling more drops is the cure‑all we all needed.
lisa howard
October 18, 2025Oh, where do I even begin? The drama of a single drop, battling the relentless tide of macular fluid, is practically Shakespearean. Picture this: a weary retina, battered by diabetic storms, suddenly finds a knight in shining carbonic‑anhydrate‑inhibiting armour. The first application feels like a cautious whisper, a tentative tap on a fragile canvas, yet the ensuing OCT scans reveal a slow, deliberate retreat of edema that feels almost cinematic. Each follow‑up appointment becomes a cliff‑hanger, with our protagonist-dorzolamide-making subtle yet decisive advances against the swelling antagonist. The patients, initially skeptical, start to hope as their vision sharpens, transforming the clinical narrative from a bleak saga into a tale of redemption. And let’s not forget the bitter taste-a small, theatrical reminder that every hero has its quirks. In the end, the real drama lies not just in the pharmacology but in the human stories that unfold when a simple eye drop becomes a beacon of possibility.
Cindy Thomas
October 18, 2025I get the excitement, but let’s keep a balanced view – the data are promising yet still limited. A cautious approach with proper monitoring is key :)
Kate Marr
October 18, 2025Patriotic eye‑drops? 🇺🇸💧💪 Let’s focus on science, not flags. 😎
James Falcone
October 18, 2025Patriotic drops? Let's keep science first.
Michael Dalrymple
October 18, 2025From a coaching perspective, it’s vital to integrate dorzolamide thoughtfully into treatment algorithms, ensuring each patient receives a personalized plan that balances efficacy with safety. Monitoring and patient education remain the cornerstones of successful off‑label use.